
The Statistician’s view of a Clinical Trial
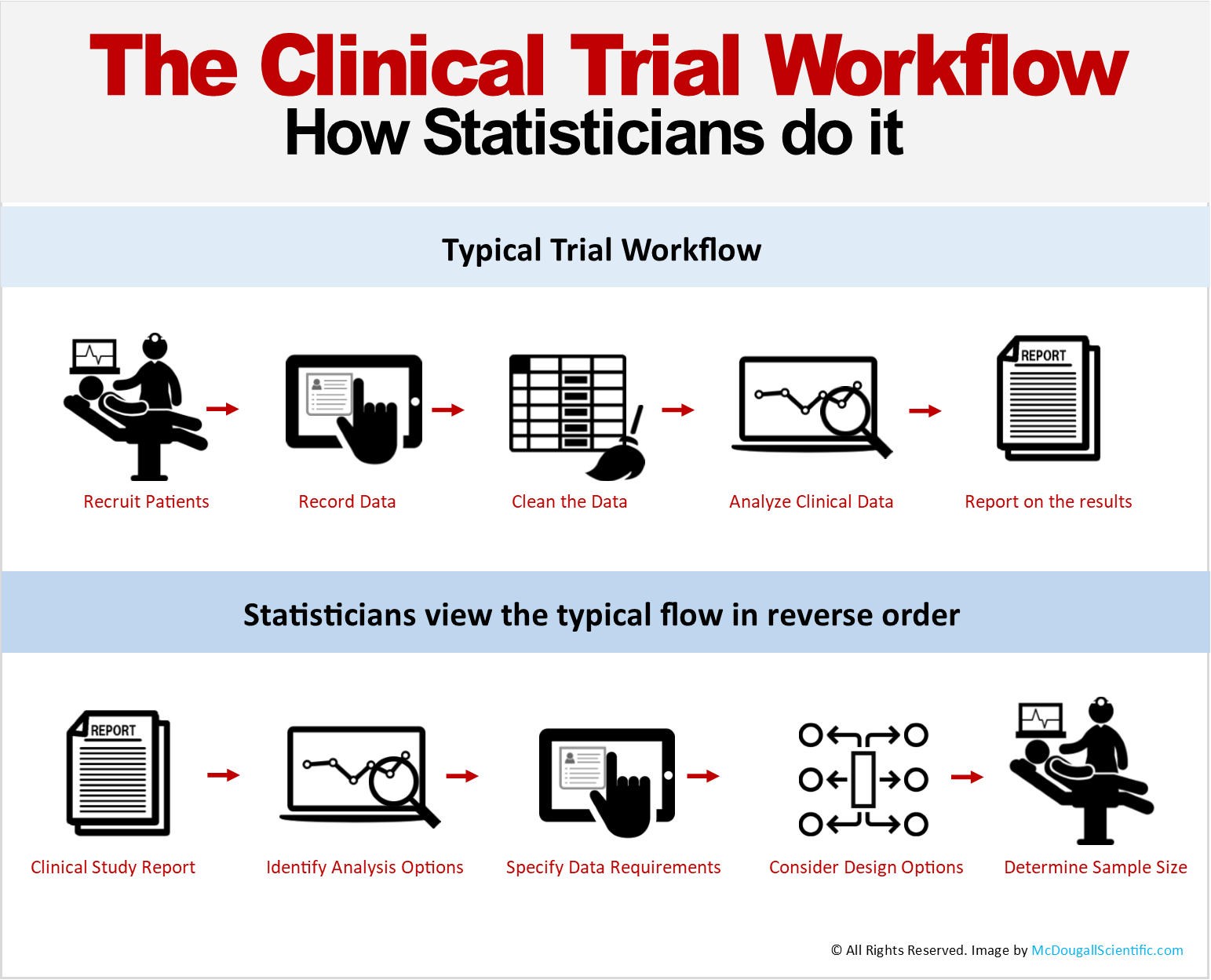
The typical flow of clinical trial data begins with the patient and ends with a clinical study report or publication. In between, data passes through a number of steps from collection to verification to analysis. Most stakeholders in clinical research view studies using this workflow and talk about milestones such as first-patient-first-visit, last-patient-last-visit, database lock, and first and final drafts of a clinical study report. Progress and performance metrics and, sometimes funding, are tied to these milestones.
Alternatively, statisticians design studies by considering the flow in reverse order. The first consideration is the research question; What questions must be answered by the study?. The best approach to formulating the research question or objective is to write down the answer to the question. That is, what would you like to write in a study report, publication, or even in the product monograph?
For example:
Approximately 45% of the treated patients were disease-free at 1 year. Among patients who were disease-free at this time point, the response was durable and lasted almost 3 years.
The statistician will then determine the best statistical analysis options that will provide results in the desired form.
For the example, to estimate the percent of patients who are disease-free, statisticians will consider estimators of proportions or, if they need to account for baseline patient attributes or covariates, they will consider statistical modelling methods. Estimating a duration requires different methods, such as estimators or models of survival or time-to-events.
Once the analysis options have been identified, the statisticians confer with the clinicians, key opinion leaders and subject matter experts to discuss the data requirements to conduct the analyses. The statistician’s role is to ensure that collected data will support the analyses. Clinicians, SMEs, and KOLs roles are to ensure the data can be collected in a reliable manner, considering factors such as memory bias, invasiveness to the patient (e.g. biopsies), and availability and capabilities of clinic staff (e.g. image adjudication).
Data requirements discussions will blend with an exchange of knowledge about the patient population which impacts the study’s design. This is an important step in the process in which statisticians will provide stakeholders recommendations for the study design to make it as efficient as possible. For example, statisticians might learn through these discussions that patients who are treatment naïve are expected to respond differently from those who are not. This may lead to handling study design differently, such as stratifying subjects to confirm this expectation. If true, appropriate design features can make statistical tests more powerful and the overall trial more efficient.
This process of consulting with clinicians, leaders, and experts to design the study and analyses can be repeated multiple times until a final study design is settled upon.
Although stakeholders often like to jump immediately to the sample size question, doing so without due consideration of the previous steps risks making decisions with faulty information. Studies have been conducted, putting patients at significant risk and at high cost to funders, that fail to answer the proper research questions or meet regulatory requirements. Sample size requirements should only be determined once the iterations of the preceding steps have settled on appropriate study objectives, with supportive data, analysis, and design, to which all stakeholders agree.
The statistician’s role is to ensure that research questions will be answered with a high degree of confidence while satisfying regulatory requirements, subject to business constraints and limiting patients’ exposure to risk.
TALK TO AN EXPERT
Set up an introductory call, have us provide you with a proposal, a review of your clinical development plan or protocol, guidance on your data, a demo of our eTMF or pricing information. Contact us. We love talking Data.